
Shiraz Mujtaba
November 8, 2023 by Jua James
EDUCATION
Masters of Science (Biosciences) – Indian Institute of Technology, Roorkee, India
Ph.D (HIV Immunology and Infection) Post Graduate Institute of Medical Education and Research, India
BIOGRAPHY
Experience
Teaching and Course Development
Research
Publication
Academic Positions
- 2014-Present: Associate Professor, Department of Biology, City University of New York
- 2010-2013: Assistant Professor, Department of Structural and Chemical Biology, Mount Sinai School of Medicine
- 2007- 2009: Assistant Professor (Research Track), Department of Structural and Chemical Biology, Mount Sinai School of Medicine
- 2004-2006: Instructor, Department of Structural and Chemical Biology, Mount Sinai School of Medicine
- 1999-2003: Post-Doctoral Fellow, Department of Physiology and Biophysics, Mount Sinai School of Medicine
Teaching and Course Development (2013-Present)
- BIO101 Introduction to Biology
- BIO201 General Biology I
- BIO202 General Biology II
- BIO211 Biotechnology and Society
- BIO461 Molecular Biology Lecture and Lab
- BIO472 Molecular Biotechnology: Theory and Applications Lecture and Lab
- BIO475 Epigenetics (course Developed)
- BIO499 Senior Seminar
Research Projects
- Understand the biology of tumor suppressor protein p53 in normal cells using small molecules to histone acetyltransferases
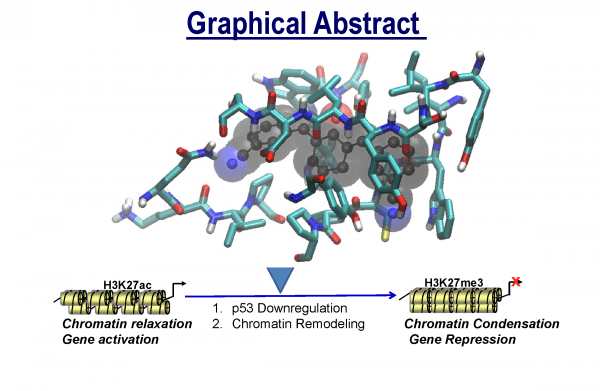 Tumor suppressor p53-directed apoptosis triggers loss of normal cells, which contributes to the side-effects from anticancer therapies. Thus, small molecules with potential to downregulate the activation of p53 could minimize pathology emerging from anticancer therapies. Acetylation of p53 by the histone acetyltransferase (HAT) domain is the hallmark of coactivator CREB-binding protein (CBP) epigenetic function. During genotoxic stress, CBP HAT-mediated acetylation is essential for the activation of p53 to transcriptionally govern target genes, which control cellular responses. cells. Collectively, HAT modulators could have the potential to reprogram the chromatin landscape and modulate biological outcomes of CBP-mediated acetylation under normal and disease conditions.
Tumor suppressor p53-directed apoptosis triggers loss of normal cells, which contributes to the side-effects from anticancer therapies. Thus, small molecules with potential to downregulate the activation of p53 could minimize pathology emerging from anticancer therapies. Acetylation of p53 by the histone acetyltransferase (HAT) domain is the hallmark of coactivator CREB-binding protein (CBP) epigenetic function. During genotoxic stress, CBP HAT-mediated acetylation is essential for the activation of p53 to transcriptionally govern target genes, which control cellular responses. cells. Collectively, HAT modulators could have the potential to reprogram the chromatin landscape and modulate biological outcomes of CBP-mediated acetylation under normal and disease conditions.
- Understand the role of Transcriptional Coactivators in the growth of Prostate Cancer
 Tumor suppressor p53-directed apoptosis triggers loss of normal cells, which contributes to the side-effects from anticancer therapies. Thus, small molecules with potential to downregulate the activation of p53 could minimize pathology emerging from anticancer therapies. Acetylation of p53 by the histone acetyltransferase (HAT) domain is the hallmark of coactivator CREB-binding protein (CBP) epigenetic function. During genotoxic stress, CBP HAT-mediated acetylation is essential for the activation of p53 to transcriptionally govern target genes, which control cellular responses. cells. Collectively, HAT modulators could have the potential to reprogram the chromatin landscape and modulate biological outcomes of CBP-mediated acetylation under normal and disease conditions.
Tumor suppressor p53-directed apoptosis triggers loss of normal cells, which contributes to the side-effects from anticancer therapies. Thus, small molecules with potential to downregulate the activation of p53 could minimize pathology emerging from anticancer therapies. Acetylation of p53 by the histone acetyltransferase (HAT) domain is the hallmark of coactivator CREB-binding protein (CBP) epigenetic function. During genotoxic stress, CBP HAT-mediated acetylation is essential for the activation of p53 to transcriptionally govern target genes, which control cellular responses. cells. Collectively, HAT modulators could have the potential to reprogram the chromatin landscape and modulate biological outcomes of CBP-mediated acetylation under normal and disease conditions.
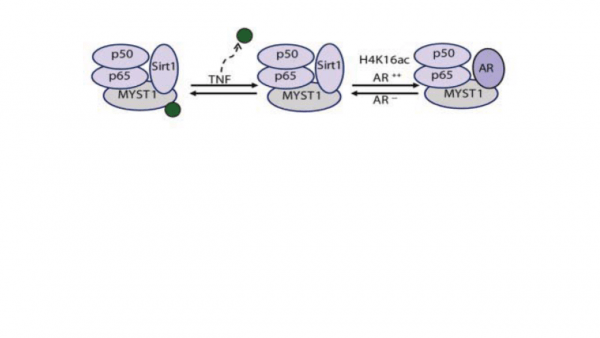
In prostate cancer (PCa), the functional synergy between Androgen Receptor (AR) and the Nuclear Factor-Kappa B (NF-kB) escalates the resistance to therapeutic regimes and promotes aggressive tumor growth. Although the underlying mechanisms are less clear, gene regulatory abilities of coactivators can bridge the transcription functions of AR and NF-ƘB. We demonstrate that activation of NF-kB promotes deacetylation of MYST1 by Sirtuin 1 (Sirt1). Further, the mutually exclusive interactions of MYST1 with Sirt1 versus AR regulate the acetylation of lysine 16 on histone H4. Notably, in AR lacking PC3 cells as well as in AR-depleted LNCaP cells, diminution of MYST1 activates the cleavage of PARP and Caspase 3 that leads to apoptosis. In contrast, in AR-transformed PC3 cells (PC3-AR), depletion of MYST1 induces CDKN1A/p21, which results in G2M arrest. Together, this study reveals that the functional interactions of MYST1 with AR and NF-kB are critical for PCa progression.
Contribution to Science
- Epigenetic Basis of Gene Regulation by Acetylation
In the late 1990s, a number of studies showed that the acetylation of lysine on histone proteins of chromatin led to the activation of downstream target genes. However, the underlying mechanism(s) of gene activation was yet to be revealed. My earlier investigations established that acetylation of lysine leads to the recruitment of acetyl lysine-binding module, namely, bromodomain, which triggers chromatin remodeling on the nucleosomes of gene promoter by the assembly of gene activator complex. During these investigations, I gained expertise in the areas of structure-function analysis, bioinformatics, and cell and chromatin biology to thoroughly understand the role of Bromodomains in activation of HIV replication and induction of growth arrest by tumor suppressor protein p53 upon genotoxic stress.
- Mujtaba, S., He, Y., Zeng, L., Yan, S., Plotnikova, O., Sanchez, R., Zeleznik-Le, N., Ronai, Z.,and Zhou, M.-M. (2004). Structural Mechanism of the Bromodomain of Coactivator CBP in p53 Transcriptional Activation. Molecular Cell 13, 251-263.
- Li S, Banck, M, Mujtaba S, Zhou MM, Sugrue MM, and Walsh MJ. (2010) p53-Induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One 5:e10486.
- Anbalagan Jaganathan, Pratima Chaurasia, Guang-Qian Xiao, Marc Philizaire, Xiang Lv, Shen Yao, Kerry L. Burnstein, De-Pei Liu, Alice C. Levine and Mujtaba S (2014). Co-activator MYST1 Regulates NF-B and AR Functions during Proliferation of Prostate Cancer Cells. Molecular Endocrinology 28:872-885.
- Define Epigenetic Mechanisms that Accelerates the Growth of Castrate Resistant Prostate Cancer
Prostate Cancer (PCa) is the second leading cause of death among men in the USA. Despite successful androgen ablation treatment, the reasons for the tumor relapse as castration-resistant PCa (CRPCa) still remain enigmatic. Recently, we showed that transcriptional coactivator MYST1 is overexpressed in CRPCa, which co-regulates Androgen Receptor (AR) and NF-κB pathways leading to metastasis. Downregulation of MYST1 function stimulates apoptosis. Further, depending upon androgen-dependent versus castrate-resistant states, MYST1 exhibits a mutually exclusive interaction with AR and histone deacetylases Sirt1. Thus, the main objective is to determine transcriptomics and genomic targets of MYST1. Our approach will identify new mechanisms that could be targeted to develop new anti-prostate cancer therapy.
- Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. (2010). Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Molecular Cell 38:662-74.
- Anbalagan Jaganathan, Pratima Chaurasia, Guang-Qian Xiao, Marc Philizaire, Xiang Lv, Shen Yao, Kerry L. Burnstein, De-Pei Liu, Alice C. Levine and Mujtaba S (2014). Co-activator MYST1 Regulates NF-ºB and AR Functions during Proliferation of Prostate Cancer Cells. Molecular Endocrinology 28:872-885.
- Pathak R, Philizaire M, Mujtaba S. (2015) Dichotomy in the Epigenetic Mark Lysine Acetylation is Critical for the Proliferation of Prostate Cancer Cells. Cancers 7:1622-42.
Publications
https://www.ncbi.nlm.nih.gov/pubmed/?term=shiraz+mujtaba
Research Support
1R01CA143662-01 (PI: Mujtaba), NCI/NIH, Title: “Chemical Modulators for p53 Functions in Transcriptional Regulation; 1R03CA143962-01 (PI: Mujtaba), NCI/NIH,Title: CBP Bromodomain Antagonists Block Androgen Receptor in Prostate Cancer; 1R03CA11471501 (PI: Mujtaba), NCI/NIH, Title: Differential Regulation of p53 function by Lysine Acetylation; CUNY ASRC (PI: Mujtaba; Co-PI: Kevin Gardner), Title: Structure-function analysis of CBP-HAT-CM354; PSC CUNY (2015) (PI: Mujtaba), Title: Unravel the Role of New Gene TPD52 in Prostate Cancer Metastasis; PSC CUNY (2016) (PI: Mujtaba), Title: Define the Role of MYST1 in Prostate Cancer Pathogenesis; PSC CUNY (2017) (PI: Mujtaba), Title: Define the Role of MYST1 in Prostate Cancer Pathogenesis; ASM Travel Award 2014; ASM Travel Award 2016; ASM Travel Award 2017; GRT18, GRT19; GRT20, PSC CUNY release time.
Poster Presentations/Publications by Undergraduate Students
Marc Philizaire, Chukuazzam Nawasike, Zimozu Orakuae, Martin Okpala, Ifeany Okpala, Olamide Fadamiro, Tajera Mcpherson, Vimal Arora, Loveth, Abdul Kamara, Fatima Lee.
Mentoring Activities
- NIH NGIMS RISE Program; 2. CUNY Research Scholar Program; 3. MSIEP Program

